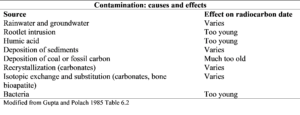Sample Pretreatment for AMS
In general, it should be assumed that all samples are affected by some form of alteration or contamination. Contaminants are carbon-containing materials that are not indigenous to the original organic material being dated. The goal of sample pretreatment is to isolate the carbon fraction required for radiocarbon dating and to remove carbon fractions that are altered or contaminated.
Selecting the appropriate pretreatment plan depends on the unique attributes of the sample itself, such as the sample type, potential contaminants, the burial context, and the size and preservation of the sample. Communication between the radiocarbon researcher and the sample collector is integral to this process.
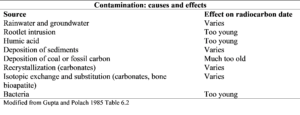
Methods Used:
Physical pretreatment
All samples are physically examined to evaluate the composition and preservation of the sample, and to determine the appropriate pretreatment plan. In many cases further physical pretreatment is required. This may involve cleaning to remove obvious contaminants and/or reduction of particle size of the sample.
Cleaning involves the physical, rather than chemical, removal of obviously intrusive materials. Some common contaminants include intrusive rootlets, which are manually separated from the sample using forceps, and surface dirt. Depending on the sample type, surface dirt may be removed by washing in an ultrasonic bath or by physically removing the outermost layer of the sample using a rotary tool or scalpel.
In some cases, samples are sieved to select an appropriate size fraction, or gently crushed to reduce the size of the particles.
Chemical pretreatment
The goal of chemical pretreatment is to remove contaminants that are chemically soluble. Certain chemical pretreatment techniques are considered routine for specific sample types or contaminants, and are described below. However, the implementation of these techniques may vary depending on the size and condition of the sample.

Acid/Alkali/Acid (AAA)
The AAA method is used to pretreat a wide variety of sample types including plant material, charcoal, wood, soils, sediment, peat, and plant-based textiles. This involves three steps: (1) an acid treatment to remove secondary carbonates and acid-soluble compounds; (2) an alkali treatment to separate out humic acids; and (3) a second acid treatment to remove atmospheric CO2. For small or poorly preserved samples, the alkali treatment may be shortened or omitted completely, or humic acids may precipitated out of alkali solution for radiocarbon dating.
The sample is placed 1N HCl and heated to 80ºC for 1 hour, centrifuged and decanted. The sample is then washed with 0.1 M NaOH to remove possible contamination by humic acids. The sample is then treated with dilute HCl, washed with deionized water and dried at 105ºC.
Hot HCl
Soils and sediments are treated with hot acid to remove carbonates and acid-soluble compounds. The sample is placed in 1N HCl and heated to 80ºC for 1 hour, centrifuged, and decanted. The sample is rinsed in deionized water and dried at 105ºC.
Collagen extraction
Collagen is a fibrous structural protein in the extracellular space in bone and tissues. The collagen fraction, with the mineral portion (bioapatite) removed, is the preferred material for radiocarbon dating bone samples when preservation permits.
The physically pretreated bone sample is broken into smaller particles, but not pulverized, to increase the surface area. The sample is treated with cold (4ºC) 1 N HCl for 24 hours to demineralize the bone. The residues are filtered, rinsed with 0.1N NaOH to remove humic acids, then rinsed with HCl to remove CO2 absorbed from the atmosphere. The sample is rinsed in deionized water to pH 4 (slightly acidic) and heated at 80ºC for 8-12 hours. The solution is then filtered through a fiberglass filter, and dried to isolate the total acid insoluble fraction (“collagen”).
Acetic acid hydrolysis (bioapatite protocol)
In cases where bone samples contain little or no collagen due to poor preservation or calcination, properly pretreated bone bioapatite can provide reliable dates if the secondary or diagenetic carbonates can be removed. An acetic acid pretreatment is used to isolate the bioapatite from tooth enamel, fully cremated bone, and poorly preserved bone samples. Bioapatite forms a relatively stable crystalline lattice, and is not soluble in weak acids. Secondary carbonates can be removed using 1N acetic acid.
An aliquot of the sample is gently crushed into ~1 mm fragments and reacted with 1N acetic acid in a flask, which is evacuated and re-pressurized periodically. The sample is allowed to react overnight. When the reaction ceases, the cleaned sample is rinsed repeatedly in deionized water and dried at 60ºC.
HCl surface leaching
This pretreatment is used to remove the exterior surface of carbonate samples that are suspected of recrystallization, exchange, or substitution. The sample is placed in a volume of 1N HCl necessary to reduce the sample weight by at least 10% in a warm ultrasonic bath until CO2 evolution ceases. The sample is rinsed repeatedly in deionized water and dried.
Organic solvent extraction
Museum preservation treatments may employ waxes, resins, oils, or glues that contaminate the organic fractions of bones or wooden objects. These materials can be removed using organic solvents such as acetone.
Isolating Carbon for AMS
After the appropriate pretreatment procedures, the carbon in the sample must isolated in the form of graphite for analysis via AMS. The carbon is first converted to a gas in the form of CO2 through acid hydrolysis for inorganic carbonates such as shell and bioapatite, and combustion for noncarbonates such as collagen and charcoal. The purified CO2 gas derived from the sample is converted to a solid, graphitic carbon for analysis.
Methods Used:
Acid hydrolysis
Pretreated carbonate samples such as shells, foraminifera, and bioapatite are reacted with 100% phosphoric acid (H3PO4) in a closed, evacuated glass vessel to produce CO2. Dissolved inorganic carbon (DIC) is produced from water samples using 85% phosphoric acid in a closed, evacuated glass vessel.
Combustion
Pretreated soils, sediments, and other organic-content materials are sealed in evacuated quartz ampoules containing CuO and combusted at 900ºC to produce CO2. Collagen samples are combusted at a lower temperature, 575ºC, in Pyrex ampoules.
Purification of CO2
The CO2 produced from acid hydrolysis or combustion is cryogenically purified from other reaction products, such as water vapor and nitrogen gas, and condensed in traps on a vacuum line using liquid nitrogen. In some cases, additional steps are required to remove other impurities, such as sulfur.
Graphitization
The purified CO2 gas derived from the sample is converted to a solid, graphitic carbon. This is achieved by reducing the CO2 in the presence of hydrogen (H2) at 580ºC in an evacuated, closed system in the presence of iron. The iron functions as a catalyst, thermal conductor, and binder which facilitates handling of the graphitized carbon. Water, which is formed as the reaction proceeds, is absorbed by magnesium perchlorate.
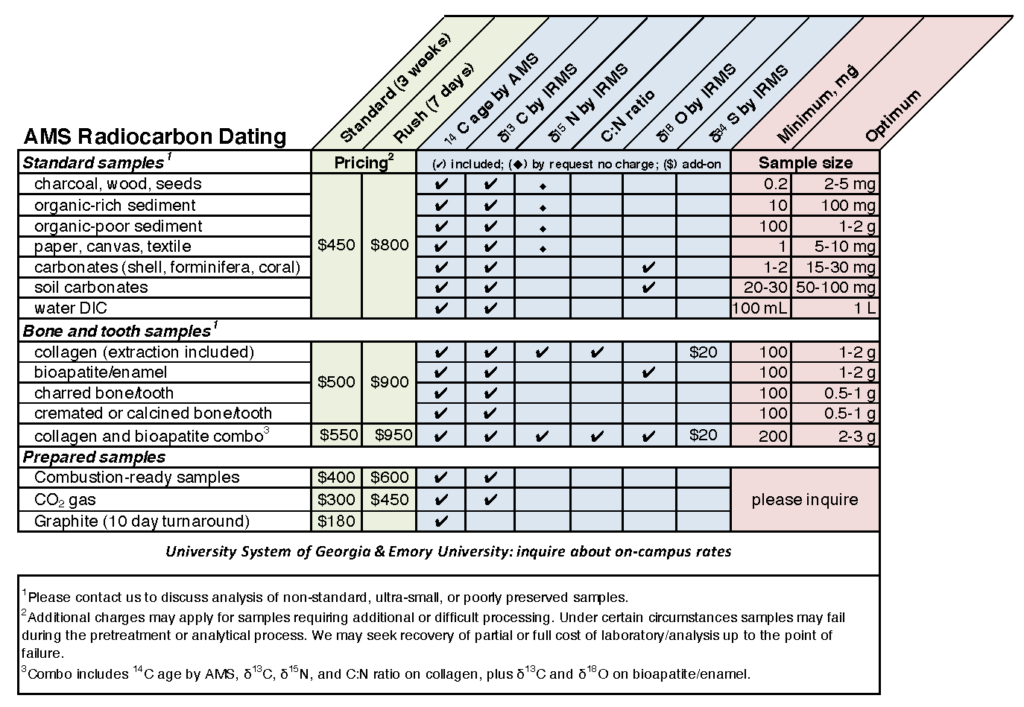
Amount of Sample Required for Analysis:
A minimum of 100 μg of graphitic carbon is required for analysis. The amount of untreated sample required to obtain this minimum size depends on the type of material being studied and the condition/preservation of the sample. Whenever possible the optimum, rather than minimum, sample size should be submitted. If you have any questions, you are welcome to contact our scientific staff concerning your samples.