Introduction to Elemental Analysis
A dust storm charges across the Sahara desert, whipping up sand and blowing it west, toward the ocean. Meanwhile, in the middle of the Atlantic Ocean, a team of scientists use filters to collect dust particles from the air, in hopes of identifying which elements are being transported across the Atlantic, from a desert thousands of miles away. These scientists will use ICP (inductively coupled plasma) technology to study the elemental composition of the dust.
Every object on earth is made of elements, from the sand in the Sahara to the snow in the Antarctic. In fact, all ordinary matter in the universe is made up of elements. Some elements will sound very familiar, such as helium, which is used to fill balloons, or sodium and chloride (aka salt), which you add to your food. Others may be a bit more obscure, such as praseodymium (used to create aircraft engines) or ruthenium (used to make jewelry). The word “element” is a term used to refer to a group of atoms that share the same defining characteristic, the number of protons in the nucleus. If two atoms have the same number of protons, but different number of neutrons, these atoms are referred to as isotopes of an element. In other words, all atoms of the element gold contain exactly 79 protons in the nucleus, however there are 19 different isotopes of gold, with the number of neutrons ranging from 90 to 126.
Atomic Emission Spectroscopy Overview
Atomic Emission Spectroscopy (AES) is also known as Optical Emission Spectroscopy (OES) and is a technique wherein elements in a mixture are identified and quantified by observing the interactions of atoms with electromagnetic radiation (e.g. light). In OES a sample is ionized, at which time electrons are excited. Upon relaxation of the excited species, energy is emitted in the form of light. The wavelength of this light is measured by a detector.
OES is based on two important principles guiding the behavior of atoms. The first principle is that atoms can only exist in discrete states, also known as levels, which are characterized by distinct amounts of energy (this is referred to as quantized energy). One way to think about this is by comparing it to living in a two-story building. Your home may be on the ground floor or it may be on the second floor, and you can live on either floor, but you cannot live in the space between. This is the same principle for atoms, and when the atom changes from one level to the next, it can either absorb or emit a photon (def.: a photon is a small packet of energy that can carry electromagnetic radiation) with energy that is equivalent to the difference in energy between the two levels. The second principle is that the frequency, ν, or wavelength, λ, of the radiation emitted or absorbed as the atom transitions from one energy level to the next is defined by the equation:
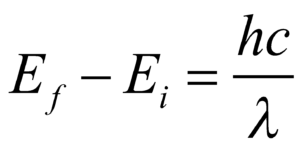
where E represents the energy of the atom at its initial ( i ) and final ( f ) state, h and c are constants, and λ represents the wavelength of the photon released. In other words, the greater the amount energy released, the shorter the wavelength of the electromagnetic radiation.
Tying these two principles together, we can see that at low temperatures the atoms of a sample of matter are essentially at their most relaxed levels, known as ground state, but these atoms can be excited to a higher energy level with heat from a flame, a plasma, or an electric arc or spark. When the atom relaxes from the excited energy level to a lower energy level, energy is released in the form of electromagnetic radiation and the wavelength of that light is inversely related to the difference in energy.
In an OES, atoms are excited in the inductively couple plasma and upon relaxation, photons are emitted. The emitted electromagnetic radiation then passes through an optical spectrometer where each wavelength is separated in space (this is the same process by which a prism separates white light into the various, distinct wavelengths and produces rainbows). The wavelengths emitted are characteristic of the atoms present, much like fingerprints. In this way, OES is used qualitatively to identify the atoms present in a sample. The intensity of the radiation can also be quantified to infer the concentration of each element.
Atomic Emission Spectroscopy – Theory
Plasma
A major component of the ICP-OES is the inductively coupled plasma. One of the first steps in ICP-OES is the introduction of sample into the plasma where it is atomized. Plasma is considered a fourth state of matter, distinct from gas, solid and liquid phases. To better understand the properties of plasma, it is useful to compare it to the other states of matter.
All matter exists in various states. In a solid, atoms are tightly and orderly arranged. When energy is added to an element in the solid state, the solid changes phase and becomes a liquid. In a liquid, the atoms adhere to a definite volume, but do not possess a fixed shape. As more energy is applied to the system, the liquid will transform in a gas. Gasses do not possess a fixed volume or shape. The atoms in the gaseous phase move about freely. When sufficient energy is applied to a gas, the atoms gain enough energy to cause an electron to detach from the nuclei. This produces a gaseous cloud consisting of positively charged ions (cations) and electrons. This final phase is identified as plasma.
An argon plasma is utilized for ICP-OES. Here, the argon ions maintain temperatures as great as 10,000 K. The energy of the plasma is capable of atomization (the conversion of a sample into atoms) and excitation of the atoms. Upon excitation, an electron from each atom transitions from the ground state to a state of higher energy. An atom in the excited state is not stable and will decay back to a less excited state. As the atom decays back to a less excited state, energy is lost by the emission of a photon. The magnitude of the photon’s energy is equivalent to the amount of energy initially required to excite the atom.
Obtaining Information
Because every element has a unique and characteristic set of energy levels, the wavelengths produced by the atoms can be used for identification purposes. In OES, a sample is introduced into the plasma where the sample is atomized and the atoms are excited. The emitted wavelengths are then analyzed with a detector for qualitative determination and the intensity of the wavelength is compared to that of standards of known concentration to determine the concentration of elements in the sample.
Caveats: Instrumental Interferences
Several types of interference occur in ICP-OES, and these can be broadly divided into non-spectral and spectral interferences. A major non-spectral interference in ICP-OES is due to easily ionizable elements, particularly group I and group II elements . In ICP-OES the goals is to provide sufficient energy to excite an electron. If more energy is supplied, however, the electron may be completely dislodged, resulting in a cation. As there is no emission of electromagnetic radiation during this process, each atom that is ionized is lost from detection. The net result is a decrease in the intensity of emission lines for that element, resulting in an observed concentration lower than actual. Another major type of non-spectral interference occurs when there is a shift in the equilibrium of the system. This equilibrium exists between the ground state atoms, excited atoms, and ions. The overabundance of an element may shift this equilibrium, affecting the intensity of emission. For example, an overabundance of potassium (K) may cause a reduction in the apparent concentration of sodium (Na). The large number of K atoms may collide with the Na atoms, resulting in an increased signal when compared to a standard solution with a considerably lower concentration of K atoms.
The two types of spectral interferences that occur in ICP-OES are background emission interferences and the overlap of lines emitted from other elements. Background emission results from the emission of excited molecules in the plasma. Molecules that are formed during the rearrangement of atoms in the plasma can absorb energy and emit light. However, unlike the discrete bands emitted from atoms, broad bands are produced when molecules emit light. The broad bands may then overlap with lines of interest. The best method for dealing with this interference is to analyze a reagent blank and subtract the signal of interferences from the sample.
The other major source of spectral interference occurs when another element in the sample matrix emits light at a similar wavelength as the element of interest. In this case, identification and quantification are not possible. One work-around is to select a different emission wavelength, one that lacks interference.
