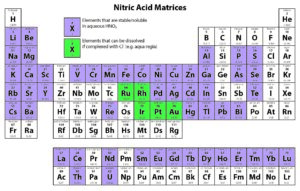
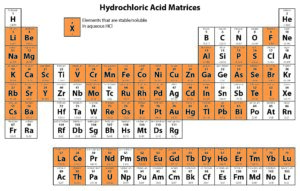
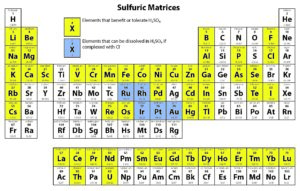
Solution-based ICP analyses require complete dissolution of the sample. Different chemical methods may need to be utilized to dissolve a sample completely. For instance, nitric acid is widely used as a digesting agent and a solvent in ICP analyses because of its oxidizing capabilities and the solubility of nitrate salts. However, platinum group metals require the addition of hydrochloric acid to promote dissolution.
The following tables provide solubility information for various elements in different matrices.